
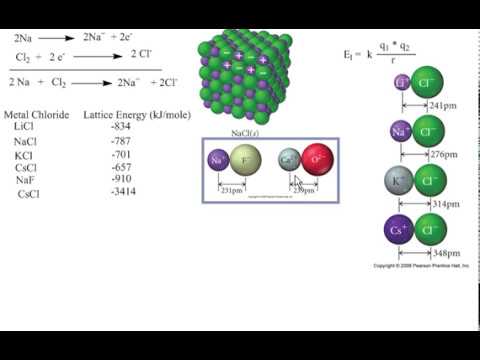
Representative values for calculated lattice energies, which range from about 600 to 10,000 kJ/mol, are listed in Table 4.2.1. The value of the constant k′ depends on the specific arrangement of ions in the solid lattice and their valence electron configurations, topics that will be discussed in more detail in the second semester. We see from Equation 4.4 that lattice energy is directly related to the product of the ion charges and inversely related to the internuclear distance. As before, Q 1 and Q 2 are the charges on the ions and r 0 is the internuclear distance. U, which is always a positive number, represents the amount of energy required to dissociate 1 mol of an ionic solid into the gaseous ions. The value lies between 0.4 and 2.00, implying that the bond type is polar covalent.\) Their electronegativity difference is 1.78. Now, compare the electronegativity difference you obtained with these three conditions to identify the bond.įor example, the electronegativity value of hydrogen is 2.20, and fluorine is 3.98. If the electronegativity difference is less than 0.4, the bond is covalent.If the electronegativity difference is between 0.4 and 2.00, the bond is polar covalent and.If the electronegativity difference is less than 2.00, the bond is ionic.Three different conditions determine the type of chemical bond that the selected elements may form.

Subtract the two electronegativity values and you will have the electronegativity difference of the two elements or atoms.
#Lattice energy trend periodic table compounds how to
How to find electronegativity? Just use a periodic table which includes it and read it for the selected element. Note the electronegativity of the first and second elements. If you want to calculate the electronegativity difference or the type of bond between two elements, you need to have an electronegativity chart for the electronegativity values of all elements on the periodic table.įollow the given steps to calculate the electronegativity or chemical bond type: If you want to learn more about the naming conventions for compounds, make sure to check our chemical name calculator. This NaCl molecule is bonded by an ionic bond whereas hydrogen (H) and hydrogen (H) bond covalently to form a dihydrogen (H 2) molecule. Instead, it tells you, if they were to form a bond, what kind of bond they would have.įor instance, sodium (Na) metal and chlorine (Cl) non-metal combine to form sodium chloride (NaCl), commonly known as table salt. 💡 You should keep in mind that this does not tell you if the selected elements actually do form a bond. We call this feature the ionic or covalent calculator. The calculator calculates the difference between these two electronegativities and then displays the type of bond that these two elements form. Select the electronegativity value of the second element. Select the electronegativity value of the first element. Follow the given steps to find out the type of bond between elements based on the electronegativity: You can also use our tool as an electronegativity difference calculator to determine the difference between the electronegativity values of elements.

The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. Electronegativity is a measure that varies between atoms and influences their chemical properties and the type of bond the atoms will form.


 0 kommentar(er)
0 kommentar(er)
